How to Raise pH in Aquarium : Best way discussion
The pH of an aquarium is an important parameter for maintaining the health of fish and the balance of the ecosystem.
Sometimes, due to natural causes or other factors, the pH value of the water decreases, which can be harmful to the fish. In such situations, it is crucial to know the correct method to increase the pH.
As an experienced aquarium hobbyist, I know how to raise the pH in an aquarium. I am confident that the methods I use will help you effectively increase the pH in your aquarium.
What Is pH
pH is a measure of the acidity or alkalinity of a solution. The full form of pH is “potential of hydrogen” or “power of hydrogen.”
p is a mathematical symbol that represents “negative logarithm,” and H is the symbol for the hydrogen ion (H⁺).
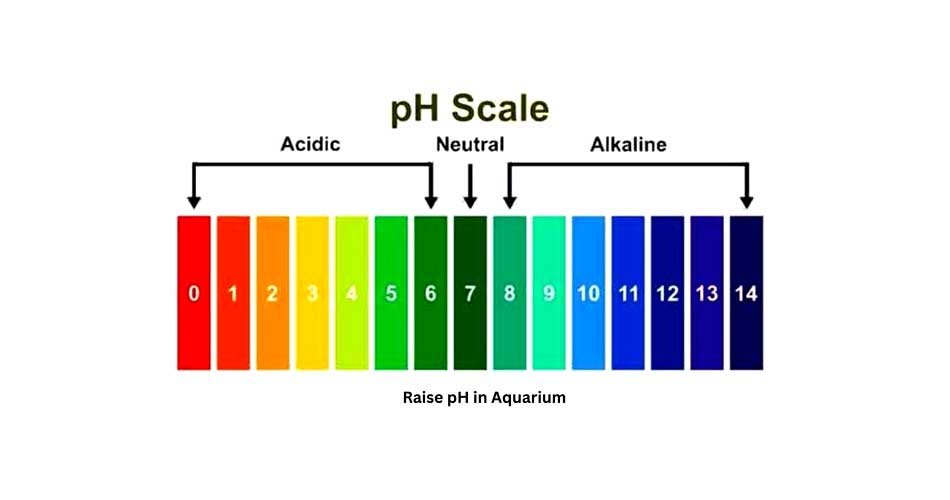
The pH value is widely used in chemistry, biology, and agriculture, with values ranging from 0 to 14.
- If the pH value is below 7, the solution is acidic and has a high concentration of H⁺ ions.
- If the pH value is 7, the solution is neutral, such as pure water.
- If the pH value is above 7, the solution is basic (or alkaline) and has a high concentration of OH⁻ ions.
The pH value affects soil, water, and the environment. It is important for plant growth and biodiversity. In an aquarium, maintaining proper pH levels is essential for the health of fish and plants.
Ideal pH Range for Different Aquarium Fish
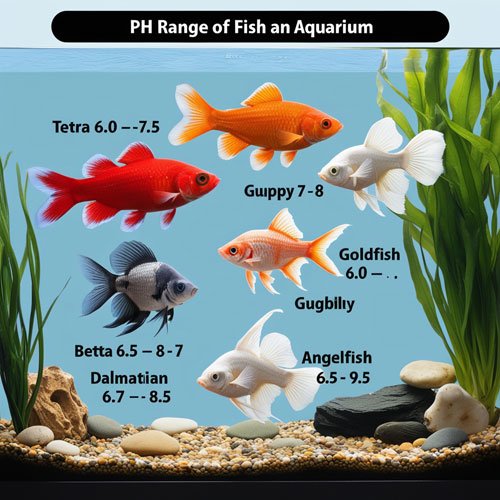
The ideal pH range for different types of aquariums depends on the type of fish and the ecosystem. The ideal pH value for freshwater fish and the ideal pH value for saltwater/marine fish can differ. Below are the ideal pH values for various species of popular aquarium fish.
Freshwater Aquarium Fish
Fish name | Ideal pH Range |
Tetra | 6.0 - 7.5 |
Guppy | 7 – 8 |
Goldfish | 6.0 - 9.0 |
Betta | 6.5 – 8 |
Angelfish | 6.8 – 7 |
Dalmatian Molly | 7 – 8.5 |
Hatchet fish | 6 – 7 |
Saltwater/Marine Aquarium
Fish name | Ideal pH Range |
Red Mandarin | 8.1-8.4 |
Blue Tang | 8.1 – 8.4 |
Ocellaris Clownfish | 8 to 8.4 |
Kole Yellow Eye Tang | 8 – 8.4 |
Gem Tang | 8.1-8.4 |
Yellow Tang | 8 to 8.4 |
Six-Lined Wrasse | 8.1 to 8.4 |
Note: pH 6.0 to 7.0 is ideal for tropical tanks, while pH 8.0 to 8.4 is suitable for coral reefs.
What Causes Low pH Levels in an Aquarium?

There are several reasons why the pH in an aquarium can drop, but in my opinion, it is mainly due to biological and chemical processes.
Below is a detailed explanation of the causes of pH drops in an aquarium:
Organic Waste Buildup
Fish feces, food scraps, and decaying plants begin to decompose in the water, producing ammonia (NH₃). This ammonia is then converted to nitrite (NO₂⁻) and subsequently to nitrate (NO₃⁻) through the process of nitrification.
Nitrifying bacteria (such as Nitrosomonas and Nitrobacter) release hydrogen ions (H⁺) when breaking down ammonia and nitrite. These hydrogen ions make the water more acidic, which in turn lowers the pH.
Excess carbon dioxide
carbon dioxide in a freshwater tank is beneficial for healthy plant growth.
However, when there is excess CO₂ in an aquarium, the water absorbs it, and the carbon dioxide, reacts with the water to form carbonic acid.
The carbonic acid (H₂CO₃) then partially breaks down to form hydrogen ions (H⁺) and bicarbonate ions (HCO₃⁻). These H⁺ ions make the water more acidic and lower the pH.
Tannins
Tannins are natural organic compounds commonly found in aquarium water from driftwood, bogwood, leaves, and certain types of substrates.
When tannins dissolve in water, they release hydrogen ions (H⁺), which increase the acidity of the water, thereby lowering the pH.
Use of Vinegar
Many times, vinegar is used to clean the glass inside the tank or to treat fish, which lowers the pH level in the aquarium.
This happens because when vinegar is mixed with water, it releases hydrogen ions (H⁺). These hydrogen ions react with the hydroxyl ions (OH⁻) present in the water to form water. This increases the concentration of H⁺ ions in the water, which lowers the pH.
Soft Tap Water
Water is classified as soft if it is low in minerals such as calcium and magnesium. This reduces the buffering capacity of the water, thereby lowering the pH.
Dirty Filters
If the filters are not cleaned properly, organic waste builds up. This increases the levels of nitrates and other acidic compounds, which lower the pH.
Not Changing the Water
If the water is not changed for a long time, the concentration of nitrates and other acidic substances increases.
This gradually lowers the pH of the water.
pH Value of the Water Source
If you collect water from a source where the pH is naturally low, the pH of the aquarium water may also be low.
What are the symptoms of low pH in an aquarium?

Fish symptoms
Symptoms of breathing problems: When the pH is low, fish suffer from breathing problems. This is mainly due to a lack of dissolved oxygen in the water and an increase in the amount of carbon dioxide. The fish then rise to the surface of the water and breathe rapidly.
Abnormal behavior: When the pH in the tank is low, the fish become restless or suddenly stay quiet at the bottom. They may also swim suddenly in a hurry.
Pale color: When the pH level is low, the fish cannot eat or breathe properly, causing them to be mentally and physically stressed. Due to stress, they cannot expend enough energy to display their normal colors, resulting in their bright colors fading.
To address this, I clean the tank with vinegar, change the water, and use other treatments as recommended by the doctor.
Reluctance to eat: Acidic water damages the gill cells of the fish. In this condition, the fish feel pain and discomfort, reducing their interest in eating and causing them to stop consuming their normal food.
Fin problems: When the pH of the water decreases, it becomes more acidic. Acidic water irritates or damages the skin and fin tissue of the fish. This can lead to problems such as rotting or cracking of the fins, known as fin rot.
Symptoms of Aquarium Water and Environment
Algae Growth: When the pH of the water in an aquarium is low (acidic), algae growth occurs. This is because a low pH means that the water has more carbon dioxide (CO₂), and CO₂ is the main nutrient for algae.
In acidic water, some nutrients, such as nitrates (NO₃⁻) and phosphates (PO₄³⁻), dissolve more easily and are readily taken up by algae. This accelerates the growth of algae.
Water Color Changes: Water can sometimes take on a milky or brownish hue because excess food debris, fish waste, or dead plants decompose, lowering the pH of the water and affecting its clarity.
Damage to Snails and Other Invertebrates: Snail shells become soft because the acid dissolves the calcium in the snail shells.
Bubble Abnormalities: Increased carbon dioxide levels can cause excess bubbles to accumulate in the water.
How to Raise pH in Aquarium

To increase the pH of your aquarium, you can follow the steps below. However, before doing so, make sure it is necessary for the fish in your aquarium to increase the pH, as some fish can adapt to a lower pH. If the fish are not having any problems, there is no need to increase the pH.
Water Change
The best method for increasing the pH of the aquarium is to change the water. This will remove other contaminants along with the low pH water in the aquarium.
Do not change more than 50% of the water at a time, as this will stress the fish.
When adding new water, make sure that the pH level of the new water is correct. I use mineral water to get the correct pH level because it naturally has a high pH. You can use it if you wish.
Change the water daily or every other day to increase the pH level quickly.
Continue this water change process until the pH level reaches the ideal value for your aquarium fish.
Baking Soda
When baking soda is added to water, it breaks down into bicarbonate ions (HCO₃⁻). These bicarbonate ions react with the excess H⁺ ions in the water. This reduces the H⁺ ions and raises the pH.
Typically, 1 teaspoon of baking soda is used for every 5 gallons of water.
Before use, the baking soda should be dissolved well in a separate container or pot and added to the aquarium gradually over a day or two.
The pH should be tested after each addition. Do not change the pH by more than 0.2–0.5 per day, as this will stress the fish. Proceed slowly to avoid sudden increases in pH.
Wait a day or two after adding the dose and then add more until the ideal pH level for your aquarium fish is reached. If the ideal pH level is reached, stop adding more.
Add Calcium Carbonate
Calcium carbonate dissolves slowly in water, especially if the water is slightly acidic. It naturally raises the pH. This releases calcium (Ca²⁺) and carbonate (CO₃²⁻) ions. The carbonate ions (CO₃²⁻) react with the excess H⁺ ions in the water and raise the pH, reducing the acidity of the water.
You can place calcium carbonate rocks (such as limestone or coral sand) in the aquarium or keep them in a bag as a decorative element in your canister filter.
Crushed calcium carbonate can be mixed directly into the water. However, it should be used very sparingly. Typically, use 1 teaspoon per 10 gallons of water.
Do not increase the pH level by more than 0.2–0.5 per day. After applying it once, wait two days before applying it again. Continue this process until the pH is ideal for your aquarium fish.
Using Raw Snail Shells
Raising the pH of your aquarium with raw snail shells is a natural and easy way to increase the pH. Snail shells are made up mainly of calcium carbonate (CaCO₃), which slowly dissolves in water to produce calcium and carbonate ions. Carbonate ions help increase the buffering capacity (KH) of the water, helping to stabilize the pH.
After collecting the snail shells, clean them thoroughly. Sterilize them by boiling them in warm water, and place them directly on the substrate or bottom of your aquarium. Monitor them regularly.
Using too many snail shells can increase the KH and pH of the water excessively, which can be harmful to your fish. You can keep 2–3 medium-sized shells in a 10-gallon tank.
pH Buffer
A simple and controlled method of increasing the pH of an aquarium is using a pH buffer. A pH buffer is a chemical compound that stabilizes the pH of the aquarium water and increases the KH (carbonate hardness) of the water. It is usually available in powder or liquid form.
Measure and apply the buffer according to the instructions on the packet. Changing the pH rapidly can be dangerous for fish and other animals. It is best not to change the pH by more than 0.2–0.5 units per day.
However, this method should not be used first. It should be used only after applying the above methods and if the pH does not increase, because any chemical compound can be harmful to your aquarium.
Sodium Carbonate
Soda ash (Na₂CO₃) is an effective chemical used to increase the pH of an aquarium and improve the KH (carbonate hardness) of the water. It dissolves in water to produce carbonate ions (CO₃²⁻), which help raise the pH.
Typically, use 1/2 teaspoon of soda ash for 10 gallons of water. Take some aquarium water in a cup or container, mix the soda ash powder in it, and dissolve it completely. It is better to make a mixture rather than adding it directly to the aquarium.
Slowly pour the soda ash solution into the aquarium water. Keep the filter or air pump running to evenly distribute the solution. Be careful not to change the pH by more than 0.2–0.5 units per day, as rapid changes can be harmful to the fish.
Note: The above methods must be applied one by one. They should not be used simultaneously.
Preventive Measures

Maintaining a stable pH in the aquarium is very important, as a drop in pH can have a negative impact on the health of the fish. You can take the following preventive measures to prevent a drop in pH:
Regular Water Changes: Change 20-25% of the water every week. Regular water changes help maintain water quality. When changing the water, make sure that the pH of the new water is the same as or close to the pH of your aquarium water.
Use Live Plants: Live plants absorb carbon dioxide through photosynthesis, which helps reduce acidity and stabilize the pH of the water. When carbon dioxide is low, the aquarium water is less acidic, reducing the risk of pH drops.
Ensure Adequate Oxygen Supply: Use a proper air pump and filtration system to ensure an adequate oxygen supply. Oxygen-rich water helps stabilize pH.
Use the Right Filter Media: Using the right filter media can help keep the pH of your aquarium water stable, as certain filter media release buffering agents into the water that reduce acidity and prevent pH from dropping.
Media rich in Calcium Carbonate (CaCO3) and Calcium Magnesium Carbonate (CMC) increase the carbonate hardness (KH) of the water. These media slowly release carbonate, which neutralizes acids and keeps the pH level stable.
Clean the Tank Regularly: Remove fish waste, dead plants, and food scraps regularly. These materials decompose and release ammonia and tannins, which lower the pH.
Cleaning the Filter: Clean the filter media, but make sure not to destroy the beneficial bacteria in the filter. Wash the filter media with dechlorinated water, as chlorine is harmful to bacteria. Dirt accumulated on the filter media increases the risk of lowering the pH, so clean it 1-2 times a month.
Use the Right Substrate: Use a substrate rich in calcium carbonate, such as crushed coral or aragonite sand. These stabilize the pH and increase alkalinity.
Choosing the Right Rocks: Place limestone or calcite-rich rocks in the aquarium. They release carbonate slowly, which helps maintain the pH of the water.
Maintain a Regular Testing Routine: Measure pH, KH, and GH regularly with a test kit. If the pH is not stable, quickly determine the cause and take action.
Ideal Aquarium Water Parameters
Maintaining aquarium water quality is crucial to ensuring a healthy environment. A proper aquatic environment not only maintains the health of the fish but also ensures the growth of biodiversity and plants. Below are the main quality parameters of aquarium water, discussed in detail:
- pH Level: The ideal value is usually from 6.5 to 8, but it depends on the fish species. For example: Amazon fish (e.g., tetras, discus): 6.0–7.0 (acidic). African cichlids: 7.5–9.0 (alkaline).
- Ammonia (NH₃): Fish waste and food decay into ammonia, which is toxic to fish. Ideal value: 0 ppm (with adequate filtration).
- Nitrite (NO₂⁻): Ammonia breaks down into nitrite, which is a toxic element. Ideal value: 0 ppm.
- Nitrate (NO₃⁻): Nitrite breaks down into nitrate. It is relatively less harmful. Ideal value: 20–50 ppm (can be harmful if higher).
- Dissolved Oxygen: The amount of dissolved oxygen in water, which is necessary for fish. Ideal value: 5–7 ppm.
- Hardness (GH & KH): GH (General Hardness) The amount of calcium and magnesium ions. Ideal value: 3–15°dGH (varies according to fish species).KH (Carbonate Hardness) The ability to stabilize the acidity and alkalinity of water. Ideal value: 3–10°dKH.
- Temperature: The correct water temperature is important for the normal physiological activities of fish. Ideal value: Tropical fish: 24–28°C. Cold water fish: 18–22°C.
- TDS (Total Dissolved Solids): The amount of minerals dissolved in water. Ideal value: 100–400 ppm.
- Chlorine and Chloramine: Chemicals in tap water that are harmful to fish. Ideal value: 0 ppm.
- Light Quality and Duration: Proper lighting is important for plants, but excessive algae growth must be prevented.
Ideal duration: 8–10 hours.
Use a test kit to test the aquarium water regularly. Adjust based on the number of fish in the tank, species, and amount of food to maintain proper water quality.
FAQ: How to Raise pH in an Aquarium
1: Why is my aquarium’s pH too low?
A: It could be due to soft water, excess waste, or driftwood releasing tannins. Over time, these can lower pH levels.
2: Is low pH bad for fish?
A: Yes, it can stress or harm fish that need higher pH levels.
3: Can plants affect pH levels?
A: Yes, live plants absorb CO₂ during the day, which can help raise pH slightly.
4: Does substrate affect pH?
A: Definitely. Some substrates, like crushed coral or aragonite sand, help raise pH slowly and steadily.
5: Do all fish need the same pH?
A: No, different species thrive at different pH levels. Always research what your fish need.